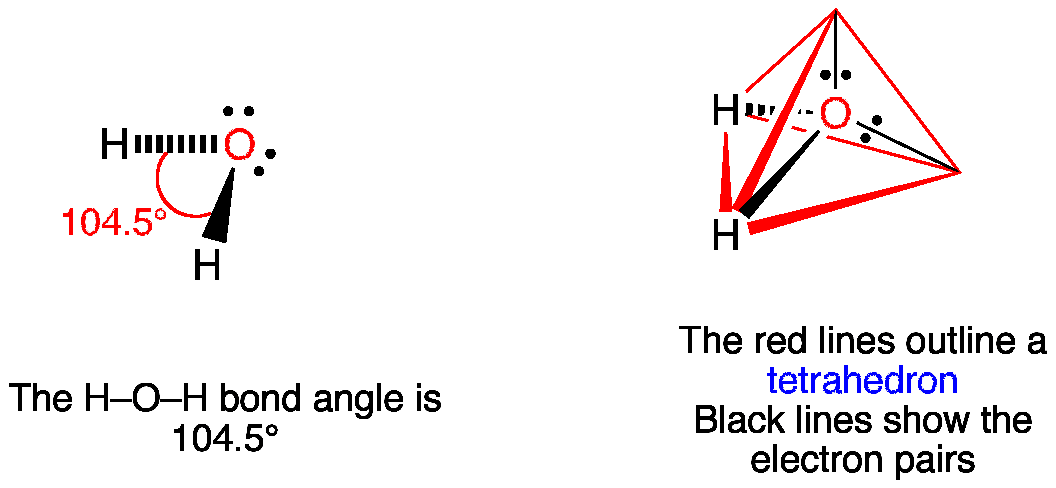
Bond Angle of H2o
Repulsion by a bond pair decreases as the electronegativity of the atom bonded to the central atom increases As the bonded atom becomes more electronegative the bonding. The bond angle in a water molecule 1045 Hybridization of H 2 O.

Bond Angles For H2o Ideal And Actual Youtube
H2O2 Bond Angle.

. The bond angle of H2O is 1045 the deviation from the tetrahedral angle 1095 the repulsion between the two lone pair of electrons. In the case of H 2 O the Oxygen the atom has 6 electrons of which two of them are bonded with a hydrogen atom leaving two lone pairs of electrons. The correct order of bond angle would be Cl2OH2OF2O As we know the bond angles are mostly governed by the repulsions of lone pairs and bond pairs of the central atom.
These two lone pairs repel the hydrogen-oxygen bonded pairs so much that the molecule is at its lowest energy arrangement. In the present case S is less electronegative than oxygen. Water or H2O has a bond length between the hydrogen and oxygen atoms of 9584 picometers.
H2O has 4 areas of electron density that repel as far as possible around the central Oxygen. Generally the molecules having tetrahedral geometry and AX2N2 notation predicted bond angle for H2O2 is 1045. The presence of these 2 lone pairs.
H20 has a bent. The two lone pairs in oxygen create repulsions that push the two hydrogens and affect their positioning resulting in a bond angle slightly below 1095. The precise bond angle is 1045Looking at the H2O.
Thus bond pairs in H2S are more away from the central atom than in H2O and thus repulsive forces between bond pairs are smaller. However there are two lone pairs of. The Lewis structure shows two single sigma bonds between the oxygen and hydrogen atoms.
Besides that these bonds. The ideal electron geometry of H 2 O is tetrahedral as there are a total of 4 electron density regions around the central O atom in H 2. In the H 2 O Lewis structure there are 2 bond pairs.
Answer 1 of 2. It also has 2 lone pairs that will repel more than the bonding pairs hence giving a bond angle of. In water the oxygen atom has two lone pairs.
When we predict the ideal bond able for H2O Water we see that it has a bent molecular geometry and expect the bond angle to be 1095 degrees. The bond angle is 10445 degrees. A quick explanation of the molecular geometry of H2O including a description of the H2O bond angles.
What is the geometry of a water molecule.

H2o Lewis Structure Molecular Geometry And Hybridization Techiescientist
H2o Molecular Geometry Lewis Structure Shape And Bond Angles

Why Are Bond Angles Of H20 And Nh3 104 5 And 107 5 Molecular Shape And Bond Angles Chemistry Youtube
0 Response to "Bond Angle of H2o"
Post a Comment